Arterex Announces Acquisition of Phoenix S.r.l., a leading Italian Medical Device Solution Provider
Written by
Arterex Medical
Published on
January 21, 2025
The transaction expands Arterex’s capabilities in medical device development, finished device assembly and packaging to Europe
(Arterex, January 21, 2025) Arterex, a leading global medical device developer and contract manufacturer, today announced the completion of its acquisition of Phoenix S.r.l. (“Phoenix”), a European developer and manufacturer of medical devices with global sales. Financial terms of the transaction were not disclosed.

As the newest addition to the Arterex group of medical device contract manufacturing companies, Phoenix brings extensive expertise in the design, development, and manufacture of disposable medical devices, along with assembly and packaging services in ISO 8 clean rooms.
“The acquisition of Phoenix is a strategic step for Arterex, allowing us to expand our medical device development and finished device assembly and packaging capabilities to Europe,” said Jeffery Goble, Arterex CEO. “With a deep commitment to innovation, quality and customer support, Phoenix will strengthen our position as a leading, global provider of specialized and multi-capability manufacturing solutions.”
Located near Modena, Italy, Phoenix develops, assembles, and packages medical devices in just over 37,000 ft2 of manufacturing space and four ISO 8 clean rooms. The company is ISO 13485:2016 certified and operates in compliance with the European Union’s MDR (Medical Device Regulation).
“We are excited to join the Arterex group of companies, given our strategically aligned missions to provide leading-edge medical device solutions,” said Marco Bulgarelli, co-CEO at Phoenix. “Arterex is a trusted leader in the medical device manufacturing sector and together we will be well-positioned to find creative and effective solutions for our clients’ needs.”
The acquisition marks the next chapter in Arterex’s journey to provide customers in North America and Europe with comprehensive services including design, development, engineering, compounding, extrusion, injection molding, tooling and mold builds, as well as advanced assembly and packaging capabilities. Arterex operates through nine state-of-the-art manufacturing facilities across three continents with over 1,250 employees and is a global leader of diversified manufacturing solutions to the medical device and life sciences industries.

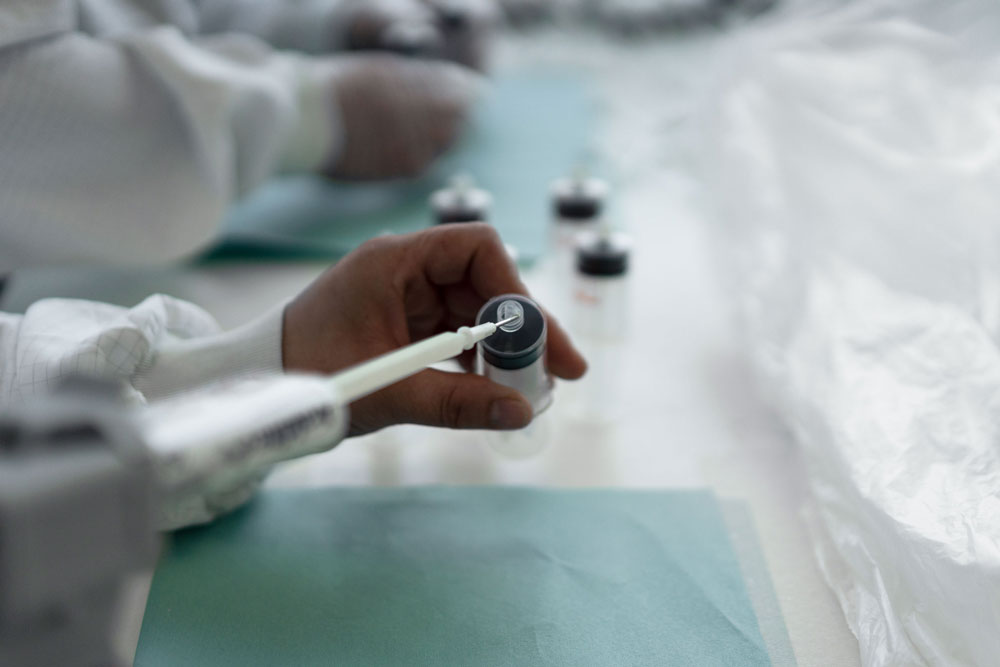
Photo caption: Located near Modena, Italy, Phoenix develops, assembles, and packages medical devices in just over 37,000 ft2 of manufacturing space and four ISO 8 clean rooms.
For further information please contact:
S!Y Communications, Inc.
Andrea Siy, President
Tel: +1 (978) 270-4080
Email: Andrea@siycommunications.com
About Phoenix
Phoenix is an Italy-based medical device developer and manufacturer. Offering product development, production, assembly and packaging services in ISO 8 clean rooms, Phoenix is known for its dedication to innovation, quality and total customer support.
Additional information is available at phoenixbiomed.it
